Phone
+91 97231 13334Rhombus Pharma

Rhombus Pharma Private Limited is the fastest growing
pharma company in Gujarat.
Rhombus Pharma Private Limited is engaged in manufacturing, marketing and exporting of Pharmaceutical Products Formulations And Nutraceuticals in many therapeutic segments in dosage forms of Tablets, Capsules, Oral Liquids, Dry Syrup, Dry Powder & Liquid Injectables, Ointments, Creams, Sachets, Nasal Sprays, Soft gels & Ayurvedic Preparations Since 1995.
Rhombus Pharma Private Limited is a well- known
pharma company in India
having more than 300 formulations in our product portfolio.
The company introduces new pharma products at every regular interval depending on market requirement. Rhombus Pharma Private Limited is engaged in providing Pharma rights by Appointing Pharma Distributors on District or Pharma Territory wise in each state of India.
More aboutOur advanced healthcare products have marked our presence in national and international regions
Marketing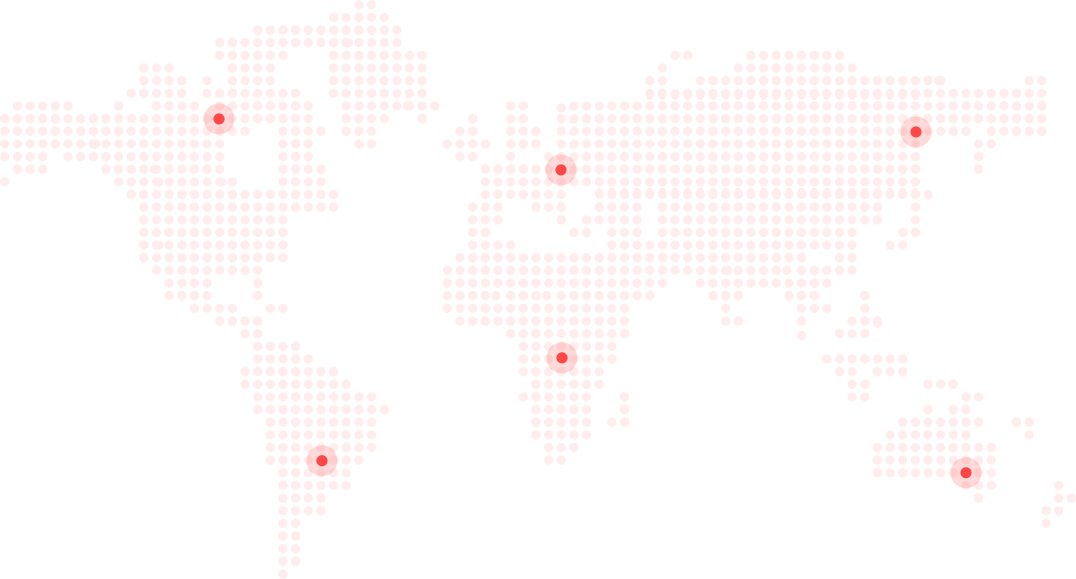
High work Achievement
Our Product Categories
ProductsWe Provide Wide Category of Products.
Newly Launched Products
NewProducts newly launched with most comprehensive research and development.
What our Clients
think about us

Stay Connected with the Latest Updates
Blog
Why Pharma is blessing for Entrepreneur?
or propaganda cum distribution is a right given to an organisation or person to sell bound products/services in a locality or a selected location.

Overview – Pharma
In simplified words, Pharma are authorization or official agreements or approvals given by Pharma Company to distributors, groups or individuals.
Why Investment in Pharma Sector is Good Choice Amid Pandemic COVID-19?
All business industries around the world have experienced a big fall due to the sudden spread of COVID-19.

History Of Pharma Companies
Pharma Companies in India have made some extraordinary progress when organizations around the world ordered business.

The Importance And Benefits Of Visual Aid In Pharma.
All marketing professionals and manufacturing pharmaceuticals use Visual Aid for recognition, promotion and business growth.